DNA mismatch repair (MMR) is a multicomponent protein complex that scans DNA to detect and correct DNA base pair mismatches and DNA insertion/deletion (indel) loops that arise during DNA replication in dividing cells. Base pair mismatches (single nucleotide variants or SNVs) occur when incorrect nucleotides are inserted into the newly synthesised daughter DNA strand and then escape the proofreading function of high-fidelity DNA polymerases. Indel loops commonly arise in the context of microsatellites - highly polymorphic repetitive DNA sequences. Alterations in the repeat length of such sequences, referred to as Microsatellite Instability (MSI), are used as clinical markers of MMR deficiency (MMR-d) in cancer patients and as such, diagnostics for immunotherapy eligibility.
DNA Mismatch Repair
How MMR Works
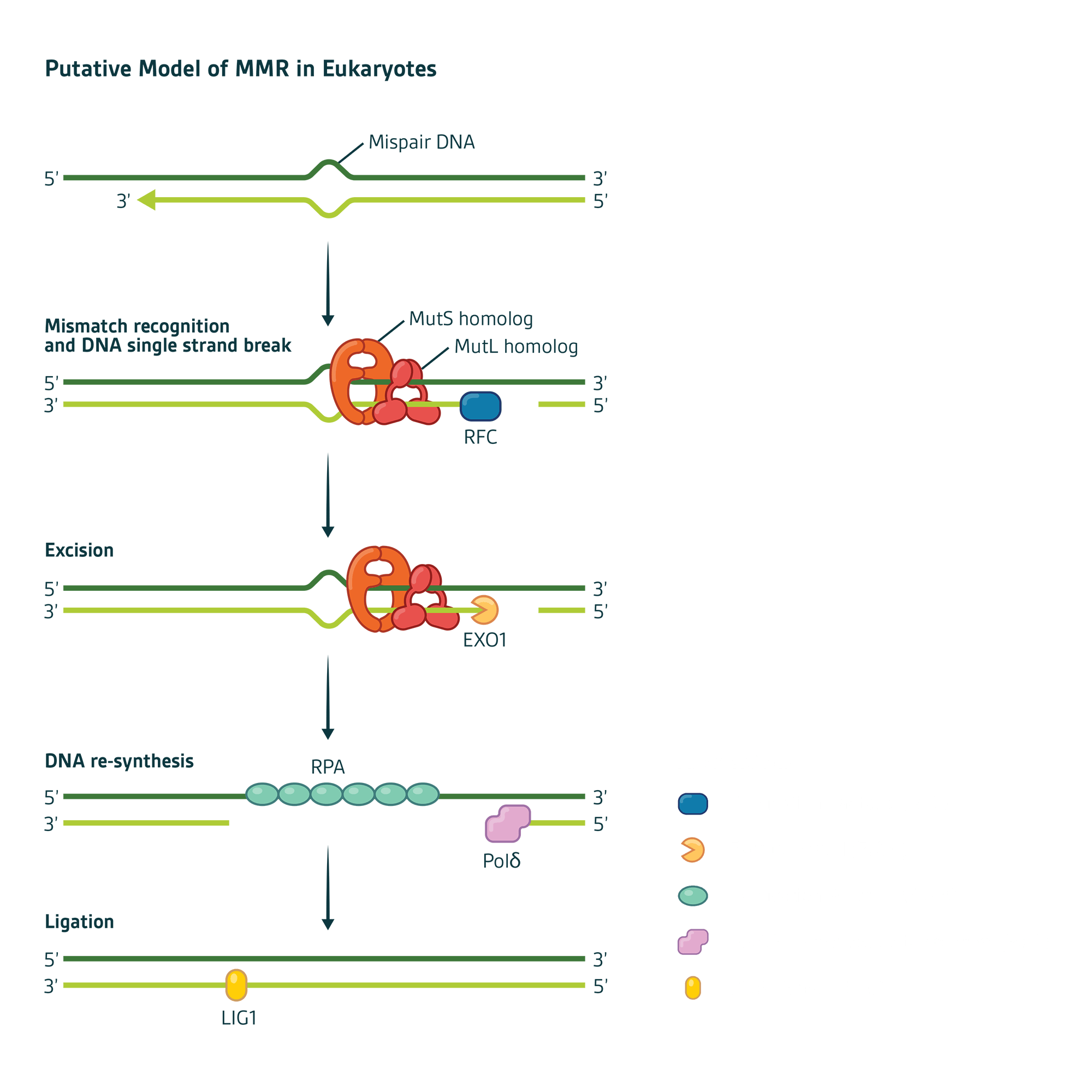
In humans, mismatch recognition by the protein complex hMutSα (MSH2-MSH6) or hMutSβ (MSH2-MSH3) initiates MMR activity. Subsequent recruitment of a second protein complex MutLα (MLH1-PMS2) results in a multicomponent protein-DNA complex whose protein-protein and protein-DNA interactions are modulated by ATP/ADP co-factors. PMS2 is unique among these proteins by virtue of an enzymatic exonuclease domain that cuts the errant daughter strand near the mismatch to initiate DNA repair. The PMS2 heterodimer partner MLH1 is a chaperone that both regulates and protects PMS2 from degradation (Figure 1).
Figure 1
Mechanistically, MMR can be divided into four steps:
1. Mismatch recognition by MSH proteins
2. Recruitment of MLH proteins that connect the mismatch recognition signal to where DNA strand scission begins
3. Excision of the errant DNA strand
4. Re-synthesis of the excision gap using the remaining DNA strand as a template